Now FDA approved to treat
cataplexy in children 6 years of age and older with narcolepsy.
How to Take WAKIX
How to Take WAKIX
WAKIX only needs to be taken once each day in the morning. Avoid taking WAKIX later in the day.
- If a dose is missed, the next dose should be taken the following morning, as soon as you wake up.
- WAKIX tablets should not be crushed or chewed.
- TAKE WAKIX
In the morning, as soon as you wake up
Once a day, every day
With or without food
- DO NOT TAKE WAKIX
Later in the day
More than once a day
Only on certain days
If you have any questions about when WAKIX should be taken, talk with your healthcare provider.
Always take WAKIX exactly as your healthcare provider has prescribed.
WAKIX comes in two tablet strengths (4.45 mg and 17.8 mg)
Depending on the WAKIX dose, more than one tablet may have to be taken.
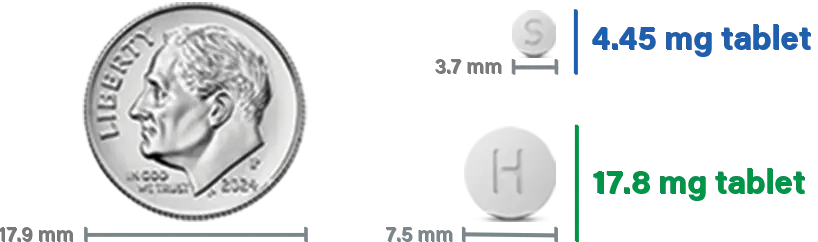
Your healthcare provider will work with you to find the right dose
Your healthcare provider will start at a lower dose and increase the dose each week to find the dose that is right for you. This process is called titration.
- Titration can take time, but it's important for you and your healthcare provider to find the dose that's right for you.
Talk to your healthcare provider about how you are feeling after starting WAKIX.
- Tell them about any improvements you notice as well as any potential side effects.
Do not stop or change the dose on your own without talking to your healthcare provider first. If you have any questions, talk with your healthcare provider.






