Now FDA approved to treat
cataplexy in children 6 years of age and older with narcolepsy.
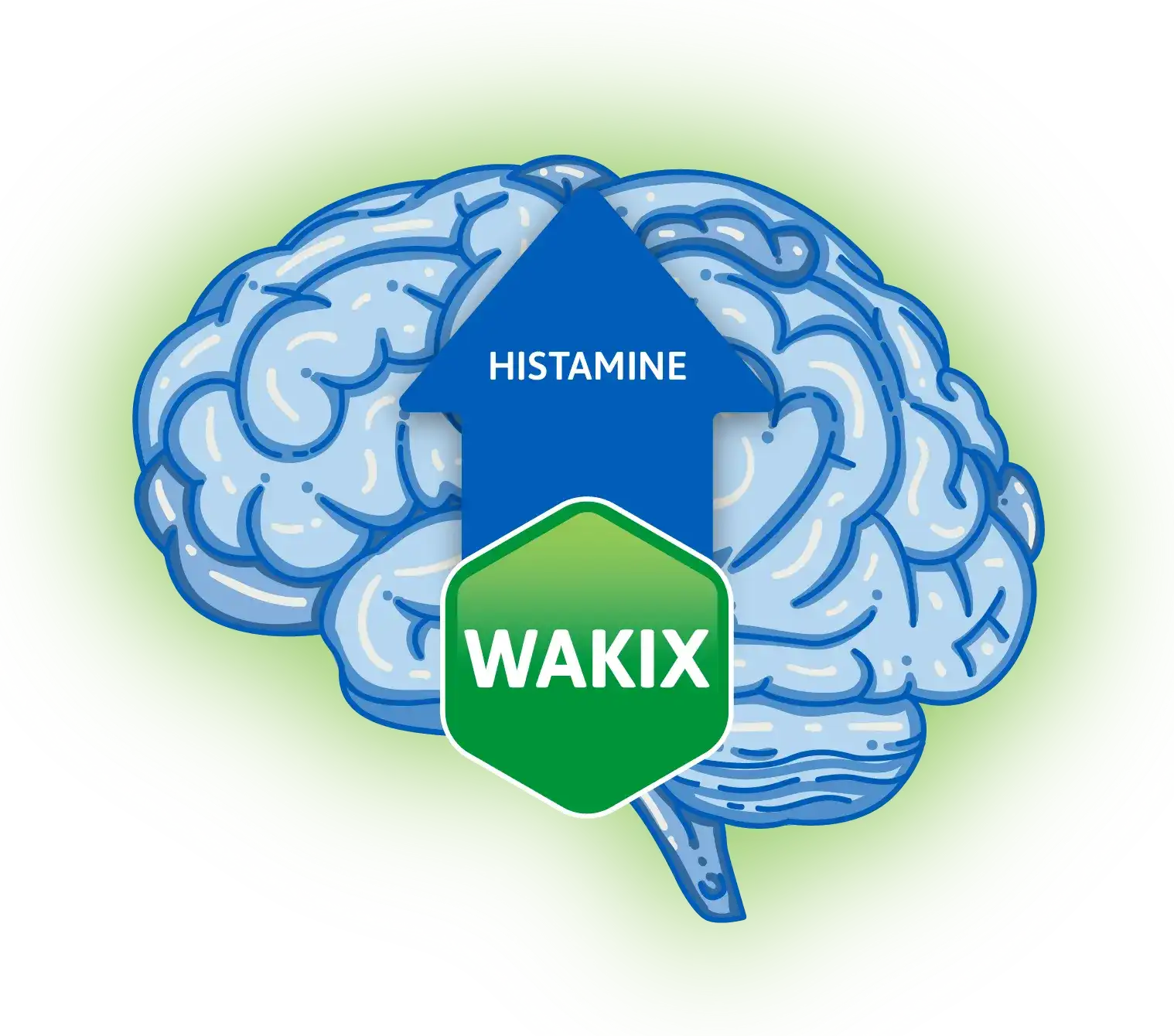
Why WAKIX?
Why WAKIX?
![First-of-its-kind icon]()
![Once daily calendar icon]()
Once-daily tablet medication
WAKIX is a once-daily tablet medication taken in the morning, as soon as you wake up.![Not a controlled substance pill bottle icon]()
Not a controlled substanceA drug or chemical that is regulated by the government based on its potential for abuse and dependence.
WAKIX is the first and onlyFDA-approved treatment for people with narcolepsy that is not a controlled substance.- In a clinical study in adults, WAKIX did not show potential for abuse, similar to placebo (sugar pill).
![Not a stimulant icon]()
Not a stimulant
WAKIX is not a stimulant, so the way your body feels when taking WAKIX may be different from medications you have taken in the past.![No significant drug interactions with sodium oxybate or modafinil icon]()
No significant drug interactions with sodium oxybate or modafinil
In a clinical study in adults where WAKIX was taken with sodium oxybate or modafinil, there were no significant effects on the levels of medications in the body.
Use this personalized discussion guide when you're ready to talk to a healthcare provider about WAKIX
Everyone has unique needs when it comes to living with narcolepsy. Use this interactive tool to answer a few quick questions and get a customized conversation plan to share with a healthcare provider.