Resources to Download
Downloads and Discussion Guides
Learn more about narcolepsy and WAKIX and find resources for talking with your
healthcare provider
Questionnaires for adults
Questionnaires for adults
Measuring your excessive daytime sleepiness (EDS)
Use the Epworth Sleepiness Scale (ESS) to assess your level of daytime sleepiness and share the results with your healthcare provider during your next discussion.
Understanding your cataplexy
If you have cataplexy or think you might have cataplexy, use these questions to understand more about your experiences and how they may be interfering with your life. Share the results with your healthcare provider during your next discussion.
Discussion guides for adults
Discussion guides for adults
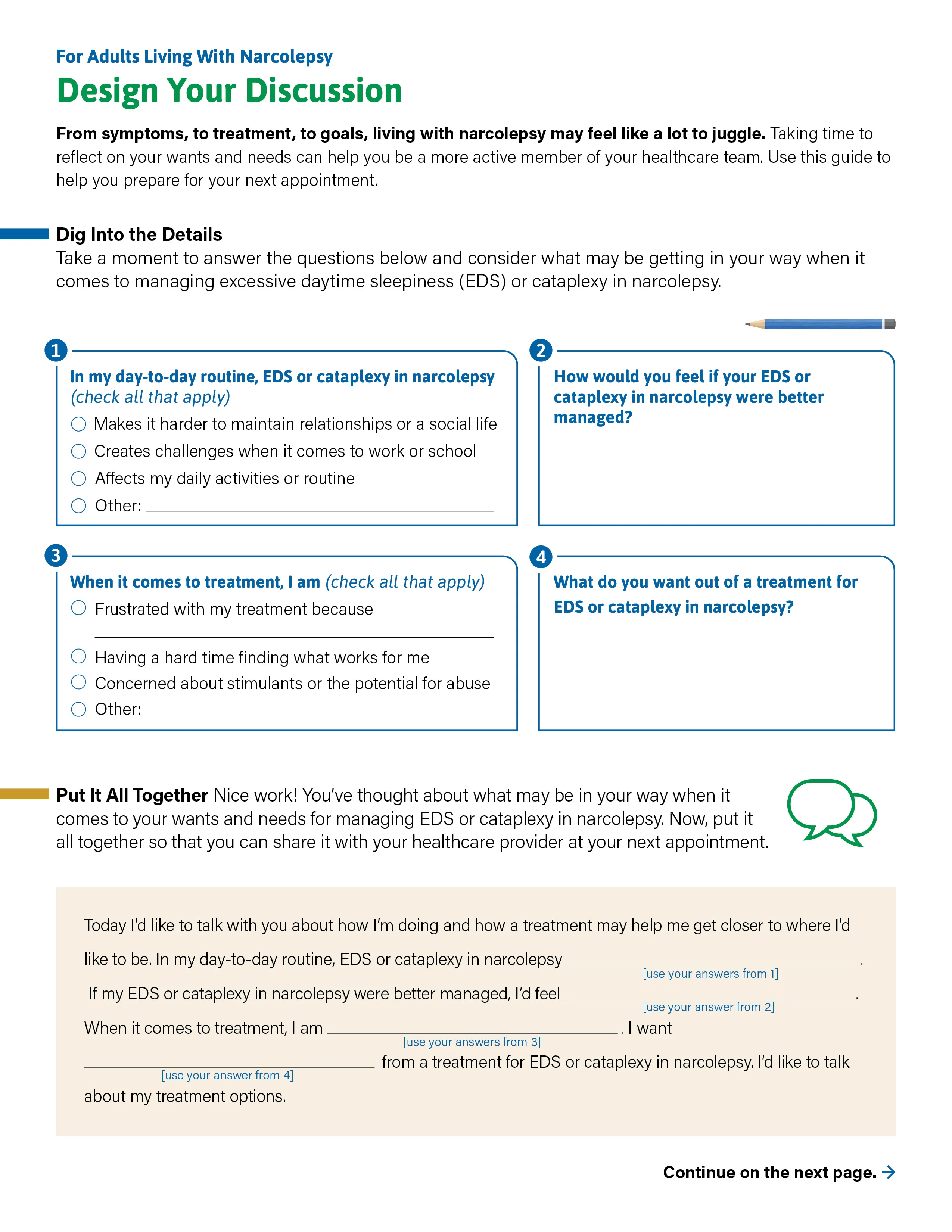
Design your discussion
When it comes to managing EDS or cataplexy as an adult living with narcolepsy, could it be time to discuss treatment options? Use this guide to reflect on your wants and needs and discuss them with your healthcare provider.
Personalize your conversation
Ready to talk to your healthcare provider about WAKIX? Use this interactive tool to answer a few quick questions to get a personalized conversation plan about your treatment needs to share at your next appointment.
Prepare for a virtual appointment
Use this guide to help navigate virtual appointments—from setting up technology to planning what you want to discuss.
WAKIX resources
WAKIX resources
WAKIX educational brochure for adults living with narcolepsy
Learn about WAKIX and how it may help you manage your excessive daytime sleepiness (EDS) or cataplexy.
Educational resource for college students or young adults living with narcolepsy
If you are a college student or young adult living with narcolepsy, download helpful information about WAKIX.
WAKIX pamphlet for parents or caregivers of children with narcolepsy
Get helpful information about WAKIX, including clinical trial results in children 6-17 years old with narcolepsy, possible side effects, and what to expect when your child is starting treatment with WAKIX.
Getting started and staying on track with WAKIX
When starting a new medication, it is important to talk to your healthcare provider so they can help you understand what to expect as an adult living with narcolepsy. This guide includes a few things to discuss with your healthcare provider when starting WAKIX.
Helping your child start and stay on track with WAKIX
When your child is starting a new medication, it is important to talk to their healthcare provider so they can help you understand what you and your child should expect. This guide includes a few things to discuss with your child's healthcare provider when starting WAKIX.
WAKIX for You overview
Navigating the reimbursement process can be challenging. For people who have been prescribed WAKIX, WAKIX for You provides the support to help get started on WAKIX. Here is an overview of the program and services available.
Tips for college students living with narcolepsy
College life comes with its own set of new responsibilities and challenges. This guide provides helpful tips on living with narcolepsy while attending school.